Counterfeit medicines are fake and of sub-standard quality that pose considerable risk to people due to contamination, lack of active ingredients or incorrect dosage of active ingredients. With more people preferring to buy medicines online, the market for counterfeit medicines is not just thriving, but the most lucrative of all global trade in falsified and illegally copied goods, netting in 150-200 billion euros (US$163 billion to $217 billion) annually.
Not only are these falsified medicines putting millions of people in harm’s way, they can potentially cause death. According to the estimates of World Health Organisation (WHO), 1 million people globally lose their lives due to falsified medicines. Missing active ingredients can render medication for otherwise curable or preventable diseases useless. In cases of fake medicines flooding the market becoming known to the public, it’s the name of the brand that comes under fire.
The number of counterfeit medicines in the market continue to rise because of more sophisticated means employed by counterfeiters to break or circumvent security measures, lack of law enforcement, complex supply chains and the rising popularity of e-commerce. According to WHO, upto 50% of the drugs bought online are fake.
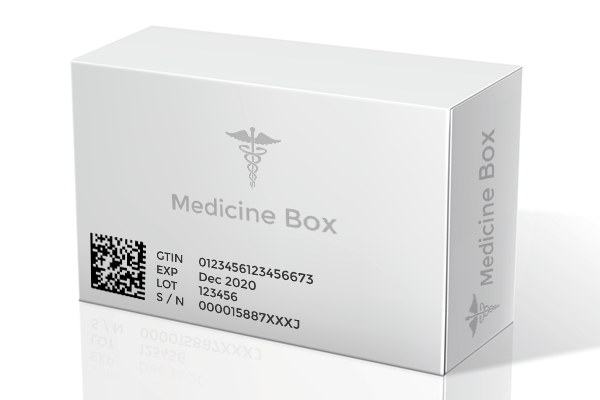
Governments around the world are turning to technology to tackle the situation by introducing regulations that maintain supply chain integrity in order to ensure authentic medicines reach consumers. The EU’s Falsified Medicines Directive, which went live In February this year, is a prime example. With its primary focus on the identification and serialization of individual packs of medicines, the regulation (1), which was issued in 2016, establishes mandatory unique identifiers on individual packs comprising of a product code, a serial number based on a randomised algorithm, a reimbursement number according to the countries which the medicine is marketed in, a batch number and expiry date.
We are at a tipping point with advancements in cloud based capabilities and technology to realize the mass deployment of smart packaging on everyday consumer products, including pharma products, as reality. It is now possible to digitize hundreds and millions of products and maintain their records on the cloud rapidly, in real time and at a low cost.
Digital tags like RFID, NFC stickers, QR codes, data matrix and barcodes can elevate a simple box of medicine as smart and intelligent by creating a digital twins of it, on an individual and batch level, allowing it to exist on the web as a digital record with its own unique and secure identity. By allowing every pharma product to have an identity on the internet, we enable an Internet of Things ecosystem of sorts, where digital tags are used to help items that could not have otherwise linked to the web on its own. These identities or digital twins do not face the barriers that their physical counterparts do. They can carry a limitless amount of information ranging from product information, geo-location to certifications on the cloud or database, facilitating a track and trace feature. The track and trace feature is the key to ensuring authentic medicines travel down the supply chain to stores. Digital tags can be scanned by smartphones and other simple reading equipment to enable supply chain partners as well as consumers to quickly pull up feed on the journey a particular medicine box has taken and verify its authenticity.
A similar feature to leverage smart packaging to deliver authentic medicines is the pharma serialization being adopted by the EU and the United States. Under this feature, each product item at an individual level contains a unique identifier in the form of a combination of numbers which is stored in a centralized hub and can be accessed through scanning a 2D barcode. The unique serial codes help sieve through fake medicines by identifying those that are genuine.
Serialization and digital twin powered track and trace features are just the tip of the iceberg with the value that smart packaging creates for businesses. They can also contribute to improved supply chain efficiencies by granting more visibility into supply chain operations. Digitization of all business operations to fight and reduce counterfeit medicines could be the prodding that pharma companies need to modernize and implement wide ranging and lasting solutions that can prise open revenue channels in quite different directions. Smart packaging on pharma products enable multitudes of potential applications ranging from smoother inventory management, higher health literacy to better clinical trial management as well as creation of richer and more dynamic customer interactions and experiences. Low cost mass smart packaging solutions also open up possibilities for using it as a conduit for two way communication with consumers, where consumers can alert authorities and suppliers to detection of counterfeit products. A digital stamp to verify genuineness will also encourage safer distribution of medicines through e-commerce platforms.
However, a digital transformation of all supply chain operations requires the partnership of all stakeholders involved. Pharma supply chains are highly complex and involve diverse participants like manufacturers, suppliers, technology providers, packaging partners and retailers. For pharma serialization or track and trace features to work, brands will need to actively seek and involve the participation and support of all its partners. As each individual product moves down the supply chain, each participant will need to scan and verify and record the events on the cloud to enable generation of accurate and real time data.
Illicit trade in falsified and substandard medicines and medical devices inflict significant damage on both consumers and brand reputation. Technological protection is the key to securing supply chain integrity in order to ensure consumers have access to safe and authentic medicines and medical devices. The investment needed to overhaul supply chain operations in order to digitize them can be an opportunity for them to modernize their businesses by leveraging existing technology. Not only will serialization and track and trace capabilities help brands thwart counterfeiters, they can be utilized to create more value by creating new channels for revenue generation. A brand that has secured its supply chain integrity, has secured its reputation. And, a brand that has an exemplary reputation for going the extra mile to protect its products from the clutches of counterfeiters, and as a result protect the health of millions of consumers, ensures its place as a market leader for the years to come.